Supplement Labels 101
The Dietary Supplement Health and Education Act (DSHEA) was created in 1994 to define and regulate dietary supplements. One of the aspects outlined in DSHEA is that Dietary Supplements must convey labeling in conformance with the appropriate provisions of federal law, including the nutrition labeling requirements, of the Title 21 Code of Federal Regulations and the Food, Drug, and Cosmetic Act.
To better understand and adhere to the set label regulations, Lief has created a supplement label template that covers the basic requirements for labeling and compliance.
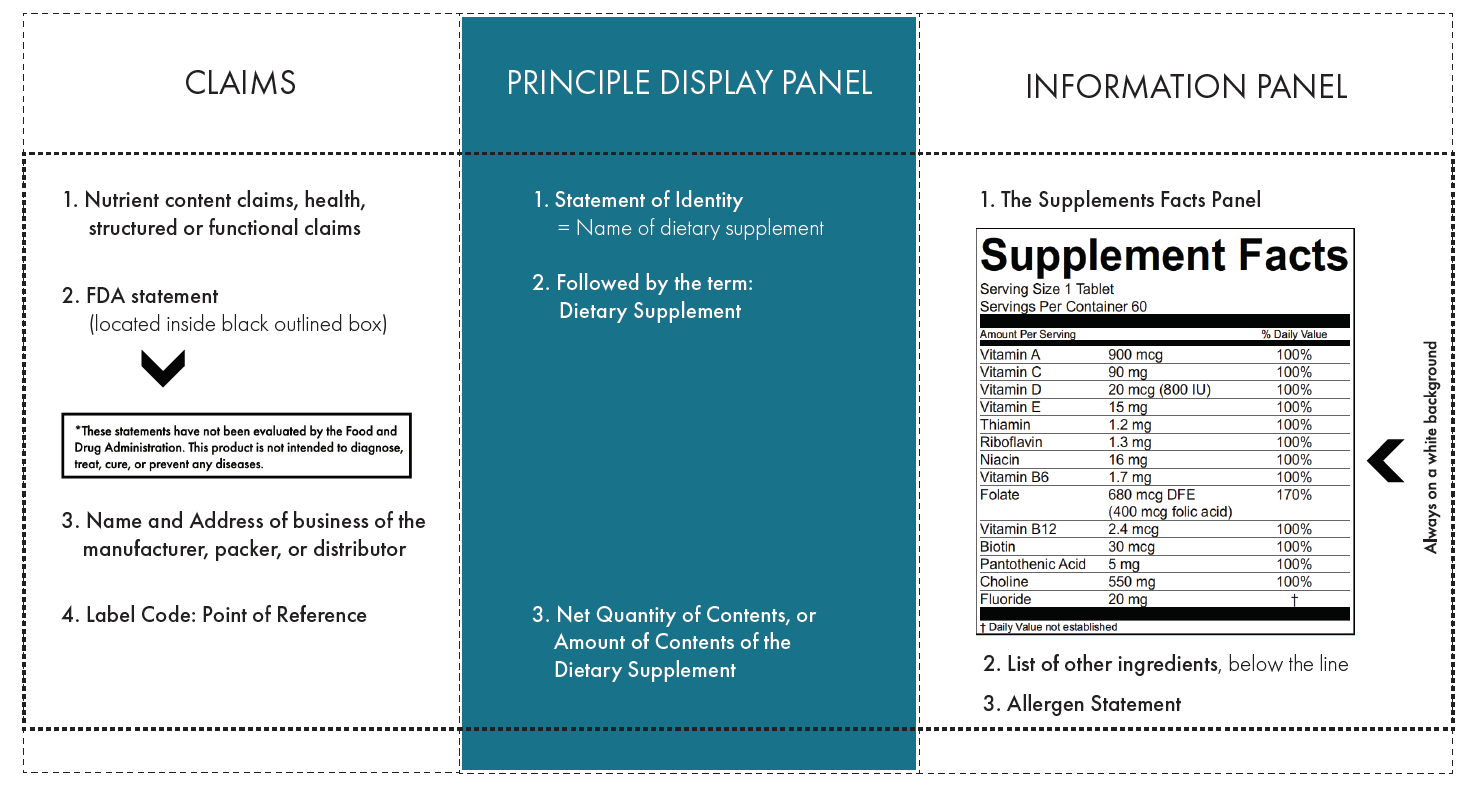
FDA-Regulated Supplement Labels Must Have:

✓ Statement of Identity
✓ Net Quantity Statement
✓ Name and Address of Manufacturer or Distributor
✓ Supplement Labeling
✓ Ingredient Statement
✓ Allergen Statement
✓ Nutrient Content Claim(s)
Element Placement
In our template, the supplement label is divided into three sections to help define where each element should be placed.
Principal Display Panel (PDP)
The PDP is the area most likely to be seen by consumers. The Statement of Identity and Net Quantity of Contents need to be prominent and easy to read.
Information Panel
The Information Panel is the area immediately to the right of the PCP and contains the rigorously regulated Supplement Facts Panel. See our template for details on SFP requirements.
Claims
Claims are located to the immediate left of the PCP and should only display nutrient, health, structure, or functional claims that adhere to the Code of Federal Regulations and meet the approval of any FDA requirements.
Click here to download Lief’s Label Template for Supplements.
Wellness Through Innovation
It is our mission to provide a seamless customer experience through innovative collaborations, highest-quality products, and unparalleled service. Get started today with a free quote.
Wellness Through Innovation
It is our mission to provide a seamless customer experience through innovative collaborations, highest-quality products, and unparalleled service. Get started today with a free quote.
Wellness Through Innovation
It is our mission to provide a seamless customer experience through innovative collaborations, highest-quality products, and unparalleled service. Get started today with a free quote.
Let's Stay Connected
Contact
-
+1.661.775.2500
-
28903 Avenue Paine
Valencia, CA 91355 USA -
Main Headquarters Hours
Monday - Friday 8AM to 5PM -
Shipping & Receiving Warehouse Hours
Monday - Friday 8AM to 2:30PM
Disclaimer: The information provided by Lief Labs on our website is for general informational purposes only. The information on the website does not constitute legal or other professional advice. All information on Lief Labs’ website is provided in good faith, however we make no representation or warranty of any kind, express or implied, regarding the accuracy, adequacy, validity, reliability, or completeness of any information on our website and/or the links provided. Lief Labs and its parents, subsidiaries, owners, shareholders, and employees are not liable for any damages arising in contract, tort or otherwise from the use of any information on our website. Because your business has its own unique needs, you should consult with legal counsel experienced in FDA, FTC and regulatory matters.
© Copyright 2024 by Lief Organics. All Rights Reserved.